Introduction:
Obesity is linked to a higher incidence of acute myeloid leukemia (AML) in older adults as well as B-cell acute lymphoblastic leukemia in children and adolescents and young adults (AYA). To date, however, the relationship between weight and clinical outcomes in AYA patients (pts) diagnosed with AML has not been clearly established. We hypothesized that obesity reflected by higher body mass index (BMI) would confer worse prognosis in AYA pts treated with intensive chemotherapy and/or allogeneic stem cell transplant (SCT) for AML.
Methods:
We conducted a single institute retrospective chart review of AYA patients aged 18 to 39 years diagnosed with AML between Jan 2006 and Oct 2022. All patients received intensive chemotherapy with cytarabine- and anthracycline-based induction and consolidation chemotherapy followed in some cases by SCT. BMI was calculated based on height and weight on the date of AML diagnosis. Additional information including European Leukemia Net (ELN) 2022 risk classification, therapy response (complete remission (CR), CR with incomplete count recovery [CRi]), receipt of SCT, relapsed disease, event-free (EFS), overall survival (OS) and percentage of pts alive at 2- and 5-years after diagnosis were collected. All data was de-identified and procured on an IRB-approved protocol.
Results:
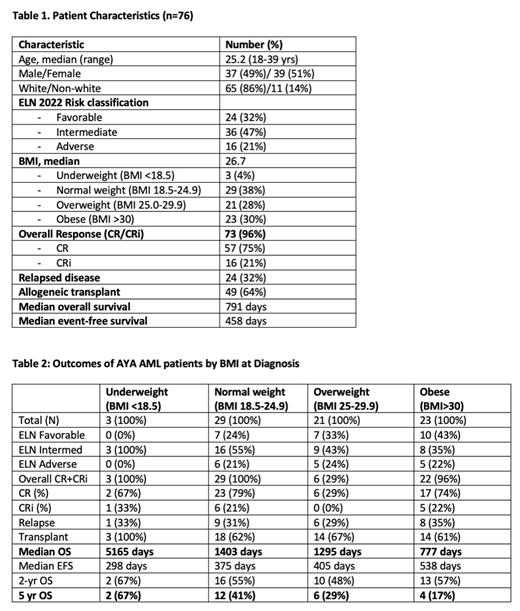
Seventy-six AYA patients were identified. Median age was 25.2 (range 18-39) years. 39 (51%) were female. The majority (65, 86%) were Caucasian. Per ELN 2022 risk classification, 24 (32%) had favorable, 36 (47%) had intermediate, and 16 (21%) had adverse risk disease. The CR rates based on ELN 2022 risk classification were 86%, 72% and 50%, respectively. Median OS for all pts was 791 days and per risk group was 1379, 569, and 595 days, respectively. Pts were then categorized per initial BMI into 4 categories. The majority of pts (44, 58%) had above average BMI (≥ 25), with 21 (28%) considered overweight with BMI 25-29.9 and 23 (30%) obese defined as BMI >30. Only 3 pts (4%) were underweight with BMI <18.5 (Table 1). Of note, 43% of obese pts had ELN favorable risk vs 33% of overweight pts and 24% of normal weight pts. Overall response rates in each BMI category were similar, ranging from 96-100% except in overweight pts where only 6 of 21 pts (29%) obtained CR. Relapse occurred in approximately one third (29-35%) of pts in the three highest BMI categories. Two thirds of all pts with BMI ≥18.5 underwent SCT. Excluding underweight pts due to small numbers (n=3), there was a trend to decreased survival in pts with BMI ≥18.5. Median overall survival among normal, overweight, and obese pts was 1403, 1295, and 777 days, respectively, with a significant difference between healthy and obese pts (p-value 0.0274 by log rank test). The percentage of pts alive after 5 years was 41%, 29%, and 17%, respectively (Table 2).
Conclusion:
Our real-world single-institute retrospective analysis suggests that AYA pts aged 18-39 yrs old diagnosed with AML and with higher BMI have decreased overall survival following intensive chemotherapy and/or allogeneic transplant. Obese patients with the highest BMI (>30) had a markedly shorter overall survival (median 777 days) and lower percentage of pts (17%) alive after five years following therapy than AYA AML pts with normal BMI (median 1403 days, 41% 5-year survival). This was despite over 40% of obese pts having favorable ELN risk. Potential reasons for this include biological heterogeneity in BMI cohorts, chemotherapy dosing, weight-related medical co-morbidities (i.e. cardiovascular disease) and/or transplant-related complications leading to shorter survival. Additional analyses to rule out the contribution of these and other factors and to confirm these results in a larger cohort are ongoing.
Disclosures
Griffiths:Physicians Educational Resource: Honoraria; MDS International Foundation: Honoraria; S. Karger Publishing: Honoraria; Novartis: Consultancy, Research Funding; Abbvie: Consultancy; CTI Biopharma: Consultancy; American Society of Hematology: Honoraria; AAMDSIF: Honoraria; Takeda Oncology: Consultancy; Astex Pharmaceuticals: Research Funding; Celldex Therapeutics: Research Funding; Blueprint Medicines, Inc: Research Funding; Genentech, Inc.: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Picnic Health: Membership on an entity's Board of Directors or advisory committees; Taiho Oncology: Consultancy; Artis Ventures: Membership on an entity's Board of Directors or advisory committees; MediCom Worldwide, Inc.: Honoraria; Alexion Pharmaceuticals: Consultancy, Research Funding; Apellis Pharmaceuticals: Consultancy, Research Funding; NextCure, Inc: Research Funding; Partner Therapeutics: Consultancy; AstraZeneca Rare Disease: Consultancy, Research Funding; Medscape: Honoraria; Vera and Joseph Dresner Foundation: Membership on an entity's Board of Directors or advisory committees. Thompson:Novartis: Research Funding; Bristol Myers Squibb: Research Funding. Wang:Sumitomo: Consultancy; Glaxo Smith Kline: Consultancy; Astellas: Consultancy, Speakers Bureau; Kite: Consultancy; Jazz: Consultancy; Daiichi Sankyo: Consultancy; Janssen: Consultancy; Gilead: Consultancy; Kura Oncology: Consultancy; Johnson and Johnson: Consultancy; Bristol Myers Squibb: Consultancy; CTI Biopharma: Consultancy; Amgen: Consultancy; Abbvie: Consultancy; Novartis: Consultancy, Speakers Bureau; NuProbe: Consultancy; Pfizer: Consultancy, Speakers Bureau; PharmaEssentia: Consultancy; Rigel: Consultancy; Sellas: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal